
Normally, an atom has an equal number of positively-charged protons as negatively-charged electrons, giving the atom an overall neutral charge. 2 + 12 = 14.Īn ion is an atom with an unequal number of protons and electrons, giving it a positive or a negative charge. A molecule of H 2O has 2 hydrogen (H) atoms, and a molecule of C 6H 12O 6 has 12 hydrogen atoms. The number in a subscript after each letter in a chemical formula indicates the number of atoms of that element in a single molecule. Which type of lever is shown in the diagram? The load is on one side and the fulcrum is located on the other.Ĩ.

Potential energy is stored energy based on position and the potential to perform work. The total energy of a system is made up of potential energy and kinetic energy. Question 6 is based on the following information and illustration.Įnergy is defined as the ability to do work. If water boils at 100☌, what is the boiling point of water on the Kelvin temperature scale? Absolute zero is the lowest possible temperature-the temperature at which all motion would theoretically cease. 0 degrees Kelvin (0K) is a temperature with a special name-absolute zero. Which of these is an exothermic reaction?ĥ. Which of these processes results in a substance with slower-moving molecules after the change?Ĥ. An endothermic reaction is one in which heat is absorbed and chemical bonds are broken.ģ. Matter can undergo physical changes to convert from one phase to another.Īn exothermic reaction is a process in which heat is released and chemical bonds are formed. Gas molecules, on the other hand, are held together by weak bonds, so gases have the least densely-packed, fastest-moving particles. The molecules in solids are held together by very strong bonds therefore, they have the most densely packed, slowest-moving particles. Solid, liquid, and gas are the three main states of matter. Questions 3-4 are based on the information and table below. This ion will have an overall positive charge if its number of electrons equals How many total atoms of hydrogen are in one molecule each of H 2O and C 6H 12O 6?Ģ.
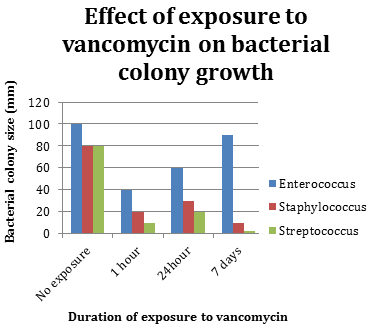
Physical Science GED Practice Questionsġ. Also check out these tips for GED Science success. Test your knowledge with these physical science GED practice questions (answer key at the end of the post). Physical science (chemistry and physics) makes up 40% of the GED Science subject test. Actual4test does not own or claim any ownership on any of the brands.By Sarah Bradstreet on in GED Practice Tests, GED Science, GED Study Guides All certification brands used on the website are owned by the respective brand owners. We offer learning material and practice tests created by subject matter experts to assist and help learners prepare for those exams. CFA® and Chartered Financial Analyst® are registered trademarks owned by CFA Institute.Īctual4test does not offer exam dumps or questions from actual exams.

The brand Cisco is a registered trademark of CISCO, IncĬFA Institute does not endorse, promote or warrant the accuracy or quality of these questions.
#Ged science practice test windows#
Microsoft®, Azure®, Windows®, Windows Vista®, and the Windows logo are registered trademarks of Microsoft CorporationĪctual4test Materials do not contain actual questions and answers from Cisco's Certification Exams. Oracle and Java are registered trademarks of Oracle and/or its affiliatesĪctual4test material do not contain actual actual Oracle Exam Questions or material.Īctual4test doesn't offer Real Microsoft Exam Questions. Actual4test doesn't offer Real (ISC)² Exam Questions.Īctual4test doesn't offer Real CompTIA Exam Questions.


 0 kommentar(er)
0 kommentar(er)
